How Do You Find The Molar Mass Of Sodium Bicarbonate
Or if you need more Calculating Molar Mass practice you can also practice Calculating Molar Mass practice problems. Sodium bicarbonate is classified as amphoteric which means it can act as both an acid and a base.
Percent Composition Empirical And Molecular Formulas Ppt Download
Sodium bicarbonate NaHCO3 is often ingested at a dose of 03 gkg body mass BM but ingestion protocols are inconsistent in terms of using solution or capsules ingestion period combining NaHCO3 with sodium citrate Na3C6H5O7 and coingested food and fluid.

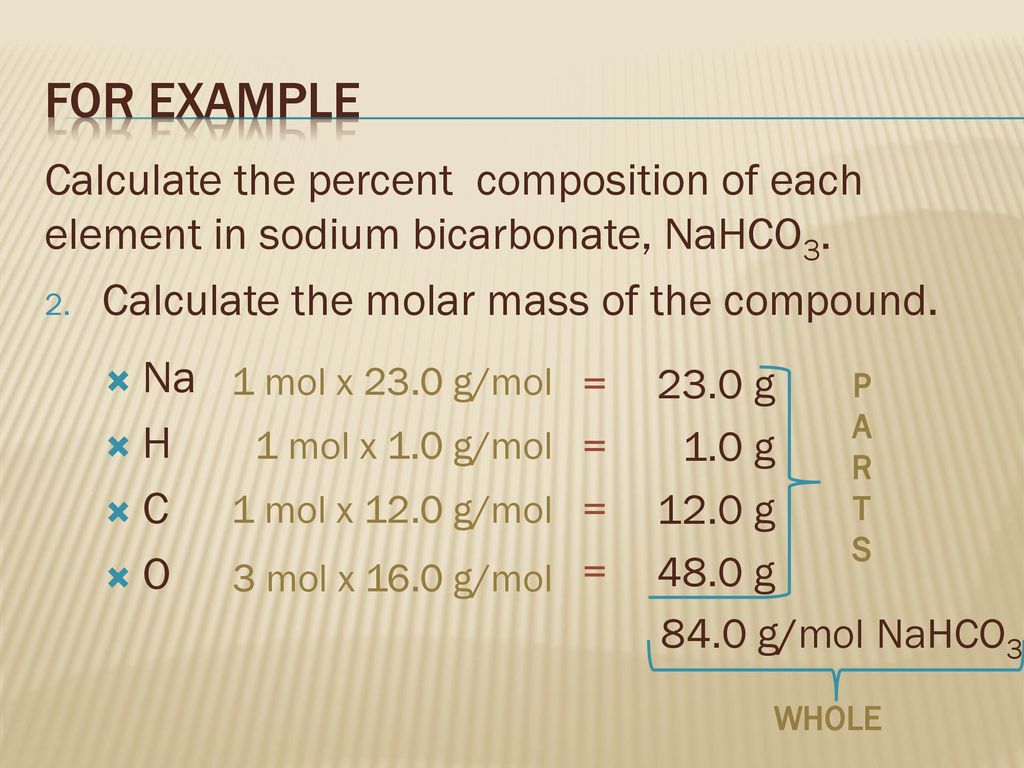
How do you find the molar mass of sodium bicarbonate. Divide the components molar mass by the entire molecular mass. 2 N a H C O N a X 2 C O X 3 C O X 2 H X 2 O. Finding the molar mass by means of calculating the sum of the atomic weight of the atoms which form the compound multiplied by the their numbers.
Once the reaction is done they heat the reaction mixture over the Bunsen. C 1201 gmol. 2020-03-01 Find the molecular mass of the entire compound.
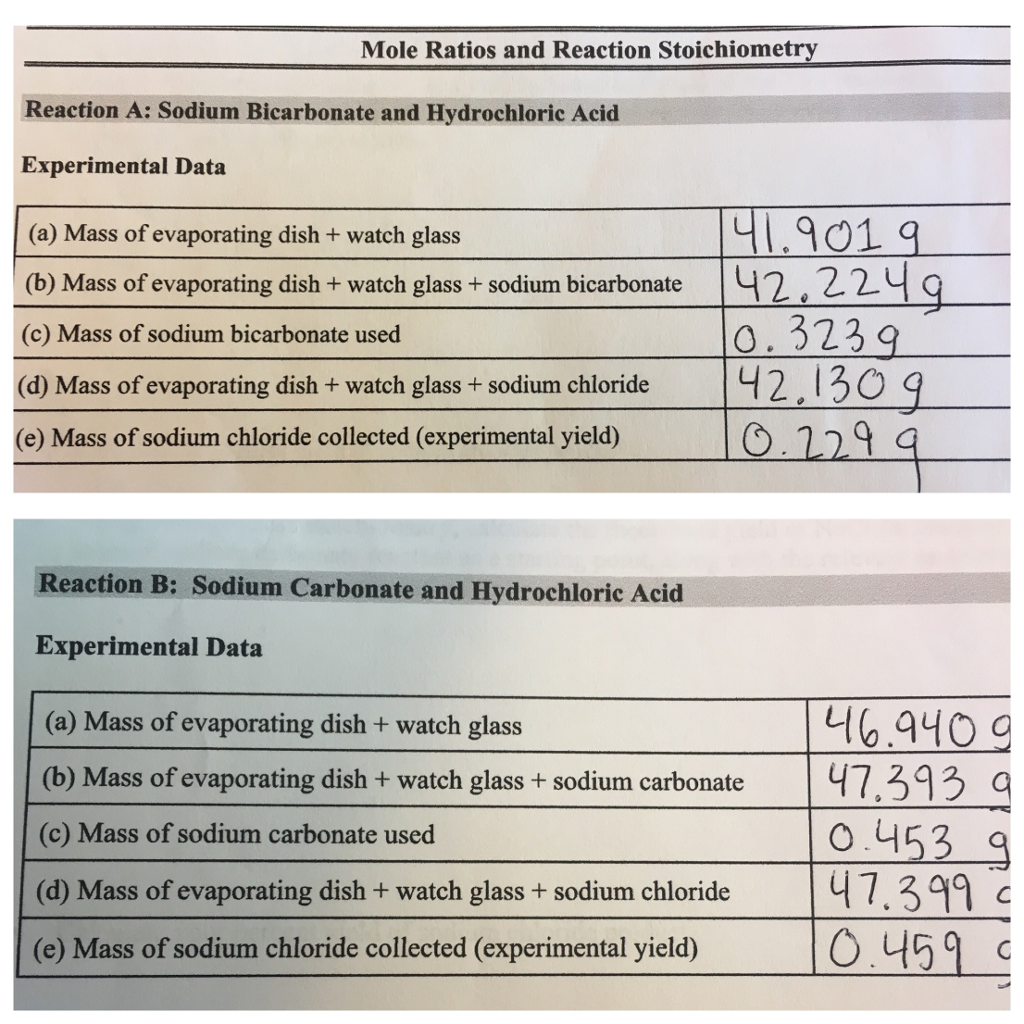
The students treat their sample of the unknown carbonate with 3 M hydrochloric acid in an evaporating dish using a watch glass to minimize splattering as the carbon dioxide bubbles up. Calcium carbonateA few things to consider when finding the molar mass for CaCO3- make sure you have the. 2 Add up the molar masses to find the molar mass of the compound.
Explanation of how to find the molar mass of CaCO3. Our tutors rated the difficulty of Determine the molar mass of sodium bicarbonate from periodi. The SI base unit for amount of substance is the mole.
Multiply it by 100 to get percent composition. 2017-02-06 The lab procedure that my class used can be found in the photo attachment. 1 grams Sodium Bicarbonate is equal to 0011903825187089 mole.
Counting the number of atoms of each element in the compound. Then you will multiply the number of moles by 6022xx1023 formula unitsmol. Also to know is how do you calculate percent by mass.
O 1600 gmol. A student analyzes an Alka-Seltzer tablet to determine the by mass of NaHCO3 present in the sample. The molar mass of a substance is calculated using three steps.
A 0500 gram tablet was powdered and mixed with 5000 mL of 0200 M HCl. 2010-03-17 One of the active ingredients in Alka-Seltzer is sodium bicarbonate NaHCO3. The two possible equations were.
More specifically it can function to neutralize both acids and. If the formula used in calculating molar mass is the molecular formula the formula weight computed is the molecular weight. The molecular formula for Sodium Bicarbonate is NaHCO3.
H 1008 gmol. Key Points The molar mass is the mass of a given chemical element or chemical compound g divided by the amount of substance mol. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by 100.
2018-10-17 Explanation of how to find the molar mass of NaHCO3. What is the difficulty of this problem. Since the formula unit CaO.
Then mass n x molar mass 094 840 gmole 8796 g. Note that rounding errors may occur so always check the results. You will now have a number between 0 and 1.
2017-10-29 There are approximately 238 moles of sodium bicarbonate in 200 g. Computing molecular weight molecular mass To calculate molecular weight of a chemical compound enter its formula specify its isotope mass number after each element in square brackets. 1 Find the molar mass of each element in the compound.
NaHCO3 is also called Sodium bicarbonate as well as baking sodaA few things. To determine the molar mass of a compound add the atomic weight on the periodic table in gmol times each elements subscript. The mole ratio is either 11 for sodium bicarbonate to sodium chloride or 12 for sodium carbonate to sodium chloride.
The purpose of the lab was to determine the equation for the decomposition of sodium bicarbonate. Definitions of molecular mass molecular weight molar mass and molar weight. The purpose of the study was to quantify the effect of ingesting 03 gkg NaHCO3 on blood pH.
We do this as follows. What is the mass of 2 moles of co2. First you need to determine the number of moles in 067 g CaO.
Na 2299 gmol. As sodium bicarbonate in Alka-Seltzer. Finding the atomic masses of elements in the periodic table.
Tablets by measuring the amount of CO 2 produced from the acid-base reaction of bicarbonate HCO 3- with acetic acid vinegar To determine the limiting reactant in the reaction between vinegar acetic acid and sodium bicarbonate by observing the effect of incremental increases in the volume of the vinegar used in the reaction Introduction. This reaction mixture was allowed to proceed until all the available sodium bicarbonate had reacted. We must first find the molar mass of sodium bicarbonate NaHCO_3.
The molar mass of a compound can be calculated by adding the standard atomic masses in gmol of the constituent atoms. Mass percentage is calculated as the mass of a component divided by the total mass of the mixture multiplied by 100. Examples of molecular weight computations.
2020-02-14 Beside above what is the mass in grams of 094 moles of sodium bicarbonate NaHCO3. 2017-02-02 067 grams of CaO contain 72xx1022 formula units.
Solved For The First Set Of Data Sodium Bicarbonate And Chegg Com

Write The Name Of The Following Compounds And Deduce Their Molecular Mass Na2so4 K2co3 Co2 Brainly In
Komentar
Posting Komentar